Intracranial aneurysm is a common type of intracranial vascular disease. Due to some factors such as congenital abnormality or acquired injury, local vascular wall is injured, which is then gradually dilated to form an abnormal bulging under the action of hemodynamic load and other factors. In human being, the morbidity rate of intracranial aneurysm is 2%~7%, and increases with age.
Coil embolization therapy is the most common method of interventional treatment for intracranial aneurysm. As proven by numerous clinical data, about 94% of unruptured aneurysm can be treated through only coil embolization. During coil embolization, the coil is implanted through micro-catheter into aneurysm cavity to block the aneurysm from blood circulation and thus occlude the aneurysm. This treatment method includes: only coil aneurysm embolization, stent-assisted coil aneurysm embolization and balloon-assisted coil aneurysm embolization.
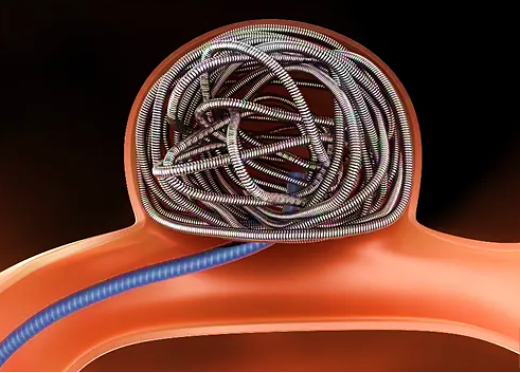
In China, intracranial coil is managed as medical device of Class III; and clinical evaluation is required. At Center for Medical Device Evaluation on NMPA, equivalent medical devices is recommended as clinical evaluation route.
On basis of subcatalogue of Catalogue for Classification of Medical Device “13. Non-active implantable device”, the clinical evaluation route is recommended:
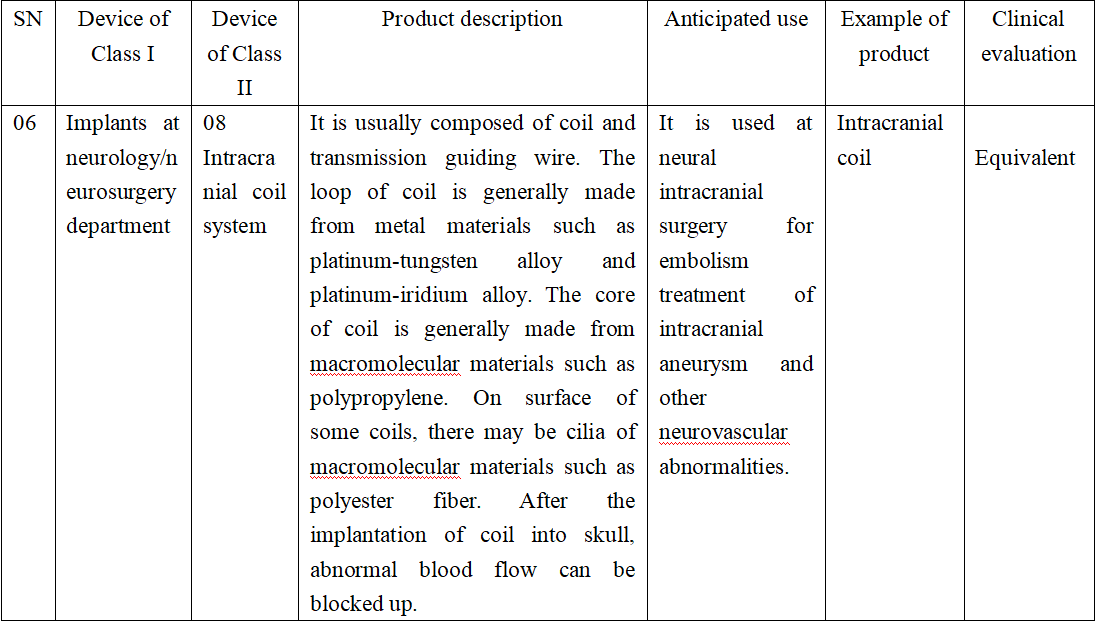
At clinical evaluation on equivalent intracranial coils, the safety and efficacy within applicable range should be illustrated according to Announcement for Issuance of Five Technical Guidelines Such as Technical Guidelines for Clinical Evaluation on Medical Device [No.73 (2021)]. Meanwhile, by combining the characteristics of this products, the following factors should be considered.
Ⅰ.Selection of suitable clinical evaluation route;
Suitable clinical evaluation route is selected according to various factors: whether the applying product is a new medical device; whether it has a product of previous generation and clinical data outside China. If applying product is a new medical device, clinical trial is required in principle. If applying product has a product of previous generation and clinical data outside China, it will be a great help for clinical evaluation.
Ⅱ.Cautious selection of equivalent medical devices;
At present, 38 varieties of intracranial coils have been registered in China. Manufacturers include Stryker, Medtronic, Johnson & Johnson, MicroVention, Peijia Medical, Microport Neurotech, Visee Medical, Ton-Bridge Medical, Wallaby Medical, Achieva Medical, TJWY Medical, Nanjing Simaide, etc. All of marketed coils are made from platinum-tungsten alloy; some coils contain cilial wire, expansive wire and gel core.
Detachment mode mainly includes: electric detachment, mechanical detachment and thermal detachment. Equivalent medical devices with same range of application and similar technical/biological characteristics should be selected. An overall consideration should be given to equivalence in various aspects: materials, structure, specification/model, detachment mode, etc. During verification of equivalence, information on equivalent medical devices should be sufficiently collected. It is a common difficult problem for clinical evaluation on equivalent medical devices to obtain performance parameters. According to our past experience, they can be collected through various official channels: patents, published literatures, evaluation reports, etc.
Ⅲ.Size/suitability/contribution of clinical data for equivalent medical devices;
Clinical data for equivalent medical devices include: clinical literatures, clinical trial data, data of clinical experiences. An overall consideration should be given to size, suitability and contribution of clinical data. It is suggested to select equivalent products which are already marketed in China and abroad, marketed for a longer time, with abundant clinical data.
Ⅳ.Sufficiency of preclinical verification;
Generally, there is a lack of clinical trial data for applying product via clinical evaluation of equivalent medical devices. Therefore, for verifying data before clinical practice, we should fully verify the safety and efficacy related to clinical practice. For example: animal experiment is one of main methods for design confirmation of safety and efficacy of medical devices; it can also be used as important supporting basis for equivalence verification. At design of animal experiment protocol, some factors should be overall considered, such as experiment objective and verification indices.
According to the public data, a bulk centralized purchase of neural intervention products has been made for several rounds nationwide. For example: Centralized purchase of coils has been implemented in Hebei Province, Jiangsu Province and Fujian Province. Our company possesses a rich experience in clinical evaluation in the field of neural intervention. In the principle of minimum burden, we help the enterprises to conduct the clinical evaluation on products and make them approved for marketing as soon as possible.
LINKS CRO
As a CRO company providing clinical trial services. LINKS CRO focus on innovation, high-risk implantation(intervention) medical device, with one-stop clinical trial solutions for the whole process of medical device research and development.The headquarters of LINKS is based in Shanghai, with subsidiary branches in Beijing, Shanghai, Guangzhou, and Shenzhen, in addition to ten regional offices. The current workforce comprises almost 200 employees, with technical personnel making up over 85% and dispersed across almost 30 cities nationwide.
Key Services include CRO, SMO, CER, Audit, Post-market research, core laboratory registration both domestically and overseas, innovation declaration, Expert Consultancy, and overseas enterprise agency services.
Key Indications include Cardiovascular, Neurovascular, Peripheral Vascular, Surgery Robot, Oncology, Ophthalmology, Medical Cosmetology, and other fields such as orthopedics. There are almost 300 projects consultations and services by LINKS each year.
