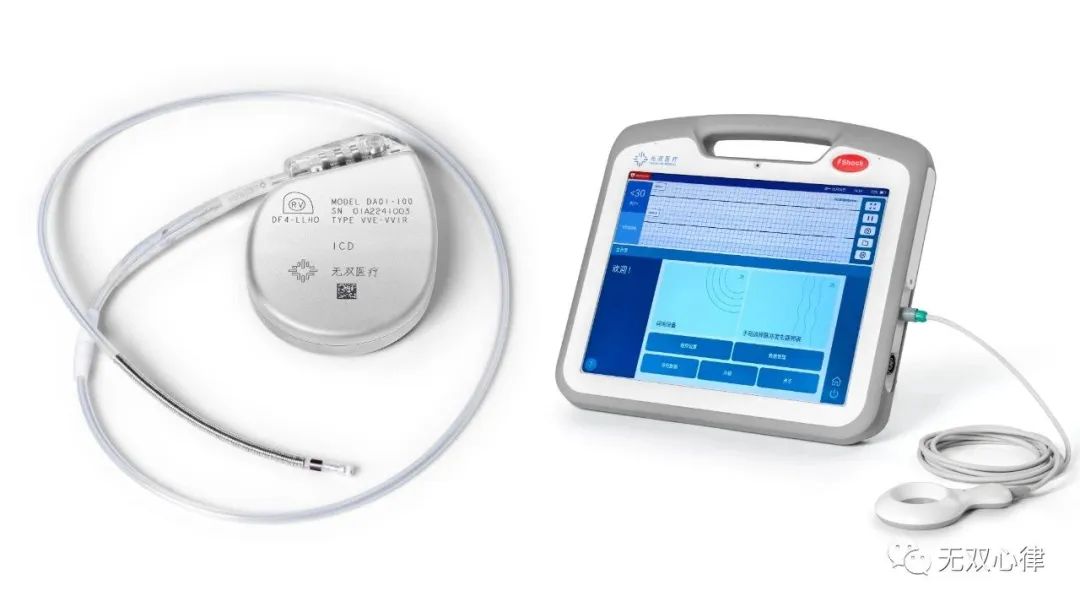
On August 23, 2023, the official website of Center For Medical Device Evaluation of the National Medical Products Administration released the results of the special review application for innovative medical devices (No. 6 of 2023).

Bringing you real-time updates on industry trends
Recently, the NMPA issued guiding principles for the registration and review f animal experimental research on medical devices, part l: decision-making principles (revised in 2021)