At the invitation of You'er Academy, Huang Yimei, Senior Medical Manager of LINKS, shared online on November 14, 2023, at 2:00 pm on the considerations of clinical evaluation pathways for cardiovascular devices. In this sharing, Manager Huang provided in-depth explanations from four aspects: clinical evaluation regulations for medical devices, clinical evaluation pathways for medical devices, consideration of clinical evaluation pathways for cardiovascular devices, and case analysis through a combination of theory and case studies.
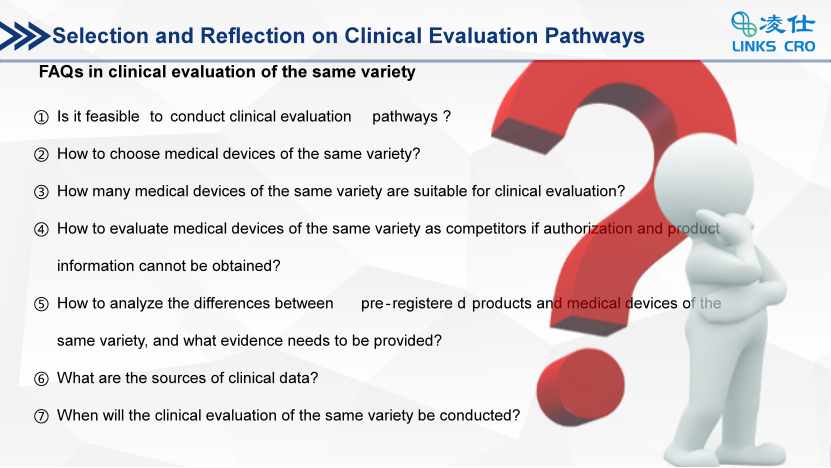




Clinical evaluation is an important step in evaluating the safety and effectiveness of medical devices, providing a scientific basis for product launch. There are significant differences in time and cost between different clinical evaluation pathways, and for the sponsor, choosing the appropriate clinical evaluation pathway is extremely important. Medical devices of the same variety include two types: equivalent devices and comparable devices, which have broad similarities with the declared product in terms of applicability, technical, and/or biological characteristics.
For products that are not included in the list of exempt clinical trials, it is necessary to clarify the product characteristics, clinical risks, existing clinical data, etc. Then, based on the relevant product clinical evaluation recommended pathways in the subcategory of the Medical Device Classification Catalog and the Guiding Principles for Decision Making on Whether to Conduct Medical Device Clinical Trials, the sponsor should choose which path to complete clinical evaluations. High-risk cardiovascular devices are small, complex and the characteristics of precision. Before referring to the Classification Catalogue of Medical Devices, it is necessary to clarify the product description and expected use of the product. In addition, the recommended clinical evaluation pathway in the document is not a mandatory standard, and a comprehensive analysis should be conducted based on the data of comparable and equivalent devices currently available on the domestic market, as well as whether pre-registered products have previous-generation products.
At the same time in the sharing, Manager Huang also conducted an in-depth analysis of multiple issues, such as how to choose the same type of medical device, how to obtain authorization for competing medical devices of the same type and related product information, and how to analyze the differences between the pre-registered product and medical devices of the same type, by explaining two cases: drug-eluting peripheral vascular stents and mechanical release spring coils.
This not only gave us a new understanding of the selection of clinical evaluation paths for cardiovascular devices but also gave us a deeper understanding of the factors that need to be analyzed and considered when choosing clinical evaluation paths.
The attendees enthusiastically interacted and asked questions in the comment section. Manager Huang provided detailed answers to more than 10 questions. This informative sharing session benefited LINKS and the attendees greatly.
The cumulative number of online participants in this training has exceeded 1200, and the cumulative number of ‘likes’ has exceeded 6000. Thank you for supporting the exchange and sharing of this training. We look forward to exploring innovative fields in the medical device industry with more outstanding individuals in the future and helping to promote the high-quality development of the medical device industry.
LINKS CRO
As a CRO company providing clinical trial services. LINKS CRO focus on innovation, high-risk implantation(intervention) medical device, with one-stop clinical trial solutions for the whole process of medical device research and development.The headquarters of LINKS is based in Shanghai, with subsidiary branches in Beijing, Shanghai, Guangzhou, and Shenzhen, in addition to ten regional offices. The current workforce comprises almost 200 employees, with technical personnel making up over 85% and dispersed across almost 30 cities nationwide.
Key Services include CRO, SMO, CER, Audit, Post-market research, core laboratory registration both domestically and overseas, innovation declaration, Expert Consultancy, and overseas enterprise agency services.
Key Indications include Cardiovascular, Neurovascular, Peripheral Vascular, Surgery Robot, Oncology, Ophthalmology, Medical Cosmetology, and other fields such as orthopedics. There are almost 300 projects consultations and services by LINKS each year.
