Clinical evaluation pathway for medical devices
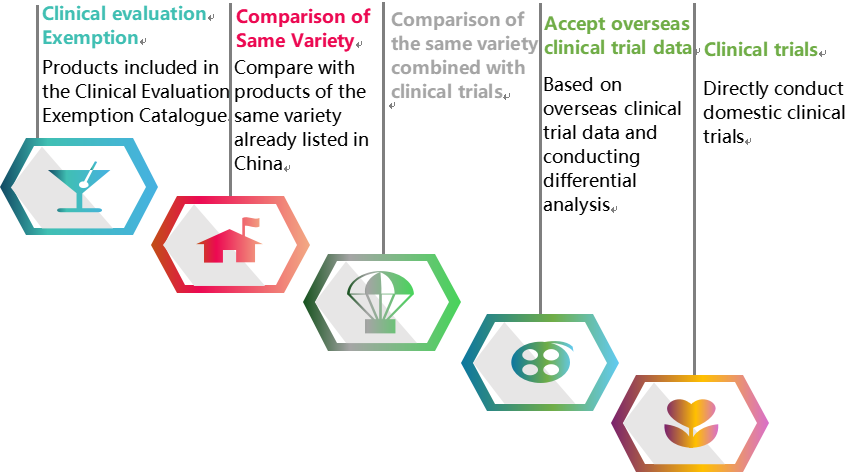
As is well known, clinical evaluation of medical devices can be carried out through multiple pathways, as shown in the above figure. The selection of clinical evaluation pathways for medical devices of different types and risk levels often troubles many regulatory staff or sponsors. Many sponsors have doubts about whether innovative medical devices will be approved for marketing after conducting domestic clinical trials. Here, we hope to provide valuable references for your decision-making.
According to the Special Review Procedure for Innovative Medical Devices issued by the National Medical Products Administration (Announcement No. 83 of 2018), the review of innovative medical devices must meet the following conditions: ①.Intellectual property rights; ②.Basic shaping; ③.Originality and significant value.
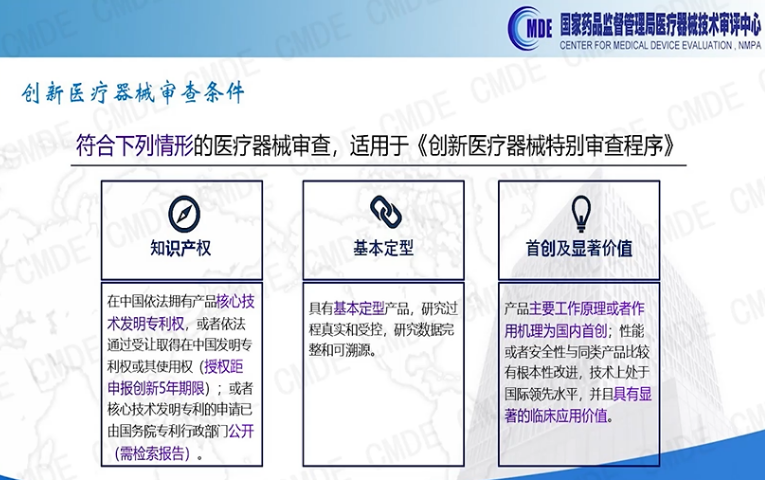
CMDE Introduction to Special Review of Innovative Medical Devices
So, here comes the question.
Is it feasible not to conduct domestic clinical trials for innovative medical devices, which not only have the core technology invention patent rights but also have the main principle or mechanism of action that is pioneering in China?
The answer is yes.
Success precedents
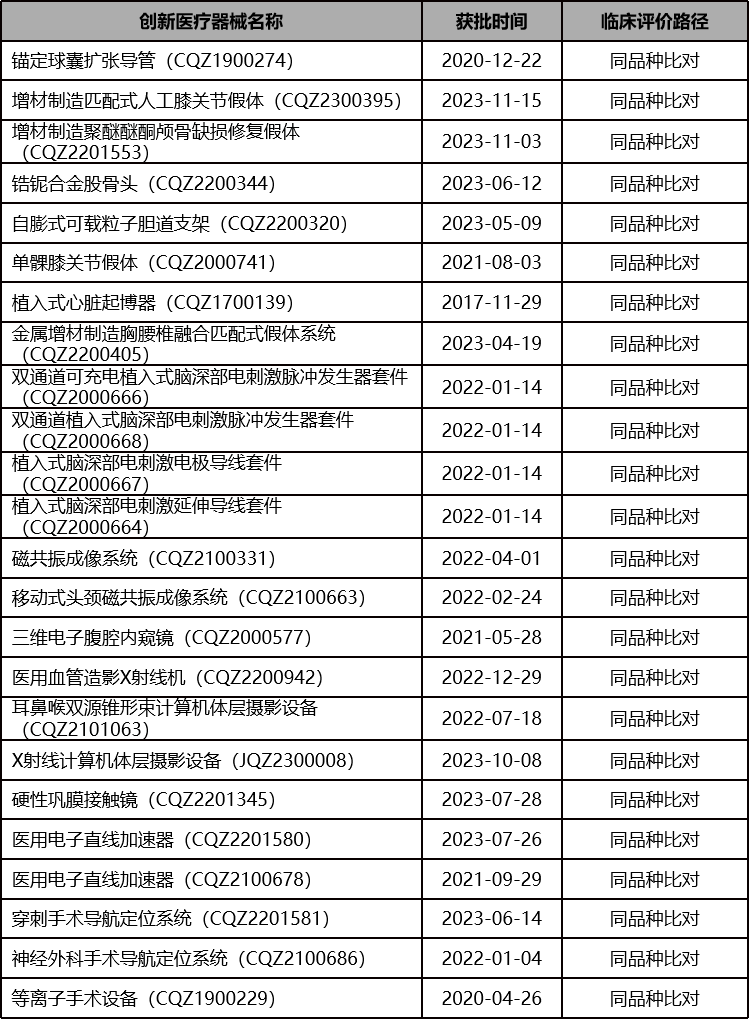
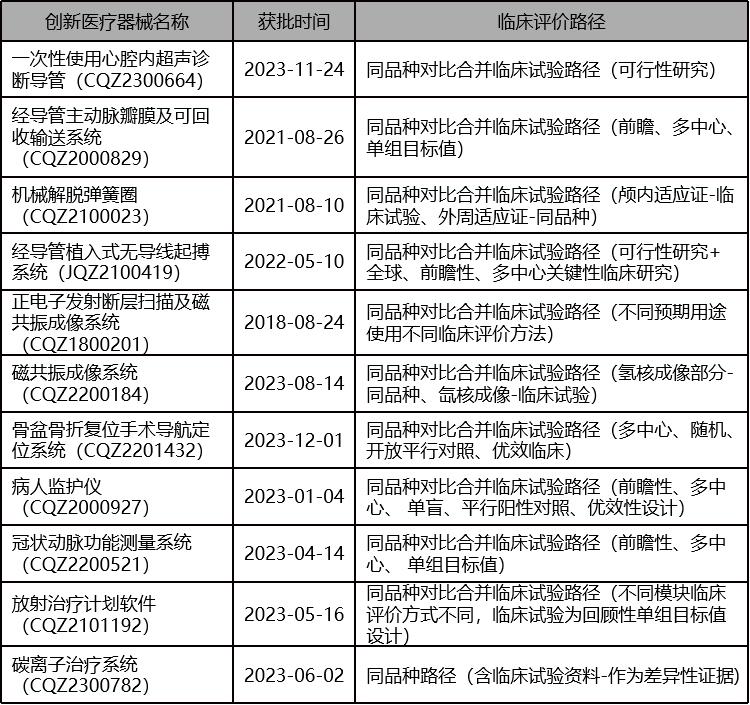
From the evaluation reports released by Center For Medical Device Evaluation (CMDE) of the National Medical Products Administration, there have been many precedents of innovative medical devices successfully registered their products through non-domestic clinical trial data: the same variety comparison pathway (CER), the same variety comparison pathway (CER) combined with other pathways, and overseas clinical trial data pathways.
同品种比对路径(CER)获批的创新医疗器械
同品种比对路径(CER)结合其他路径获批的创新医疗器械
接受境外临床试验数据路径获批的创新医疗器械
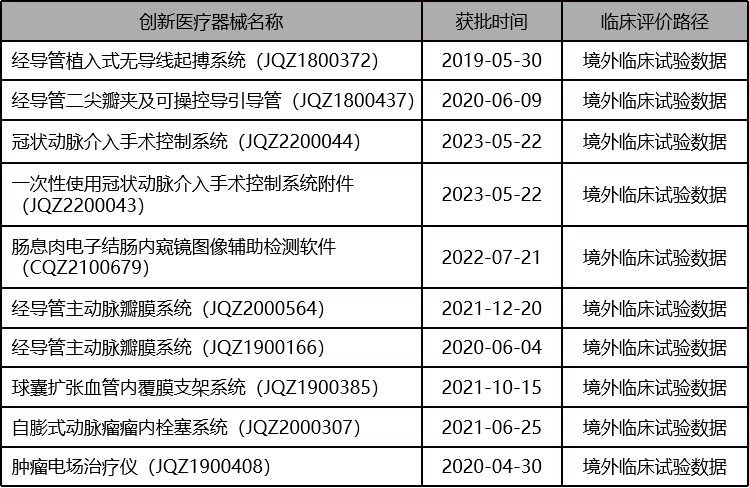 Is it somewhat unexpected? These are all examples that have been tested and successfully achieved through practice. It turns out that so many innovative medical devices have not undergone domestic clinical trials, and a considerable portion have only been approved through comparison with the same variety.
Is it somewhat unexpected? These are all examples that have been tested and successfully achieved through practice. It turns out that so many innovative medical devices have not undergone domestic clinical trials, and a considerable portion have only been approved through comparison with the same variety.
These cases provide us with valuable experience: even for innovative medical devices, when choosing a clinical evaluation path, it is not necessary to make a one-size-fits-all decision but to consider the product characteristics, clinical risks, existing clinical data, and other factors comprehensively.
Key points of clinical evaluation path for innovative medical device decision-making:
The difficulty in applying for innovative medical devices through nonclinical trial pathways lies in how to demonstrate the safety and effectiveness of their innovative points. The key point is:
· Innovation of the product: The higher the level of innovation of the product, the lower the possibility of choosing a comparison path with the same variety.
· Clinical risk of the product: The higher the clinical risk of the product, the more comprehensive clinical evaluation is needed, such as conducting clinical trials.
In the following situations, innovative medical devices can consider comparing or combining clinical trials of the same variety or overseas clinical trial pathways:
· There are previous generation products or similar products with high similarity: differences can be demonstrated through non clinical trial data to demonstrate safety and effectiveness, such as performance confirmation, performance comparison, performance verification, biological evaluation, model testing, computer simulation testing, animal testing, etc; Or combine feasibility clinical studies or clinical trial data to demonstrate that the pre-registered product can achieve its expected performance with acceptable risks.
· Having overseas clinical trial data: The pre-registered product has overseas clinical trial data and complies with the Technical Guidelines for Accepting Overseas Clinical Trial Data of Medical Devices. The sponsor can register and apply according to the path of accepting overseas clinical trial data.
Brief Summary
When drafting clinical evaluation pathways, it is necessary to conduct an in-depth analysis of the pre-registered product, combine key factors such as product characteristics, clinical risks, and existing clinical data, and flexibly choose the most suitable clinical evaluation pathway or combination of pathways to ensure that the safety and effectiveness of medical devices are fully verified.
If there are previous generation products or similar products with high similarity, or if the pre-registered product has sufficient overseas clinical trial data, it is recommended to prioritize using the same variety or a combination of multiple pathways for clinical evaluation. At the same time, sponsors should fully leverage the process advantages of innovative medical devices, actively communicate and cooperate with regulatory authorities, and make full use of existing data resources to choose the most effective clinical evaluation path, while ensuring that standards are not lowered and safety is guaranteed, to reduce the burden on enterprises, accelerate the launch process of innovative medical devices, and benefit more patients.
Please pay attention to our next introduction for specific case studies of innovative medical devices. Links has rich experience in clinical trials, clinical evaluations of similar products, and registration. Whether you encounter confusion in the selection of clinical evaluation strategies or the specific implementation process, please feel free to communicate and discuss at any time. You can contact us and we will be happy to provide you with professional and efficient solutions.
LINKS CRO
As a CRO company providing clinical trial services. LINKS CRO focus on innovation, high-risk implantation(intervention) medical device, with one-stop clinical trial solutions for the whole process of medical device research and development.The headquarters of LINKS is based in Shanghai, with subsidiary branches in Beijing, Shanghai, Guangzhou, and Shenzhen, in addition to ten regional offices. The current workforce comprises almost 200 employees, with technical personnel making up over 85% and dispersed across almost 30 cities nationwide. Key Services include CRO, SMO, CER, Audit, Post-market research, core laboratory registration both domestically and overseas, innovation declaration, Expert Consultancy, and overseas enterprise agency services. Key Indications include Cardiovascular, Neurovascular, Peripheral Vascular, Surgery Robot, Oncology, Ophthalmology, Medical Cosmetology, and other fields such as orthopedics. There are almost 300 projects consultations and services by LINKS each year.
