On August 18, 2021, with the close cooperation of academician Hu Shengshou and Professor Pan Xiangbin of Fuwai Hospital of Chinese Academy of Medical Sciences, Lux Valve implantation was successfully completed for two patients with tricuspid regurgitation. So far, Lux Valve, a global innovative product independently developed by Jenscare, has completed the enrollment of all patients in confirmatory clinical trials. This is the most cutting-edge research progress made in similar products in the world and an important milestone of Jenscare's flagship products, which means that the safety and efficacy of Lux Valve have been further confirmed.
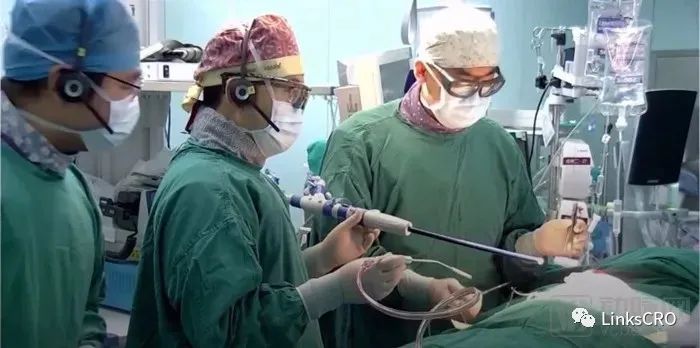
(Professor Pan Xiangbin is undergoing tricuspid Lux Valve implantation)
It is understood that 8 top medical institutions in China participated in the confirmatory clinical trial. In addition to the aforementioned team of Academician Hushengshou and Professor Panxiangbin, they also included the team of Director Xuzhiyun and Professor Lufanglin from Shanghai Changhai Hospital; The team of Director Meng Xu、Professor Zhanghaibo fromBeijing Anzhen Hospital; The team of Director Dongnianguo and Professor Shangxiaoke from Union Hospital,Tongji Medical College,Huazhong University of Science and Technology;The team of Professor Guoyingqiang and Professor Chen Mao from West China Hospital,Sichuan University; The team of Professor Yang Jian from the First Affiliated Hospital; The team of Professor Guohuiming from Guangdong Provincial People's Hospital;The team of Secretary Wang Jian'an and Professor liuxianbao from the Second Affiliated Hospital Zhejiang University School of Medicine. From the feasibility clinical trial to the completion of confirmatory clinical trial, the safety and efficacy of Lux Valve have been further confirmed, marking that China's transcatheter tricuspid valve replacement products and treatment technology have taken the lead in the world.
Links Medical Scientific (Shanghai) Co., Ltd.(LINKS CRO) serves as the CRO (contract research organization) in this research process. We would like to thank the sponsor and researchers for their full support.
Links cro is always committed to solving problems for customers and creating value for the enterprise!
【Artical From】Artery Network
LINKS CRO
As a CRO company providing clinical trial services. LINKS CRO focus on innovation, high-risk implantation(intervention) medical device, with one-stop clinical trial solutions for the whole process of medical device research and development.The headquarters of LINKS is based in Shanghai, with subsidiary branches in Beijing, Shanghai, Guangzhou, and Shenzhen, in addition to ten regional offices. The current workforce comprises almost 200 employees, with technical personnel making up over 85% and dispersed across almost 30 cities nationwide.
Key Services include CRO, SMO, CER, Audit, Post-market research, core laboratory registration both domestically and overseas, innovation declaration, Expert Consultancy, and overseas enterprise agency services.
Key Indications include Cardiovascular, Neurovascular, Peripheral Vascular, Surgery Robot, Oncology, Ophthalmology, Medical Cosmetology, and other fields such as orthopedics. There are almost 300 projects consultations and services by LINKS each year.
