On September 13, 2023, the “Public Welfare Training on Medical Device Production Enterprise Registration, Clinical Trials, and Clinical Evaluation,” hosted by the Beijing Municipal Medical Products Administration, organized by the Beijing Institute of Medical Device Testing and undertaken by LINKS Medical Scientific (Shanghai) Co., Ltd.(LINKS CRO), successfully concluded. This training invited authoritative experts from the Beijing Municipal Medical Products Administration and senior experts from Links for online exchanges, covering three topics: “Preparation of Research Materials for Passive Medical Device Registration,” “Interpretation of Quality Management Norms for Medical Device Clinical Trials and Supervisory Situations,” and “Selection and Reflection on the Clinical Evaluation Pathway for Medical Devices.” The training aimed to help registration applicants of medical devices better understand relevant professional knowledge, requirements, and workflow of medical device registration, and improve the quality and efficiency of registration applications.
First Training丨Preparation of Research Materials for Passive Medical Device Registration
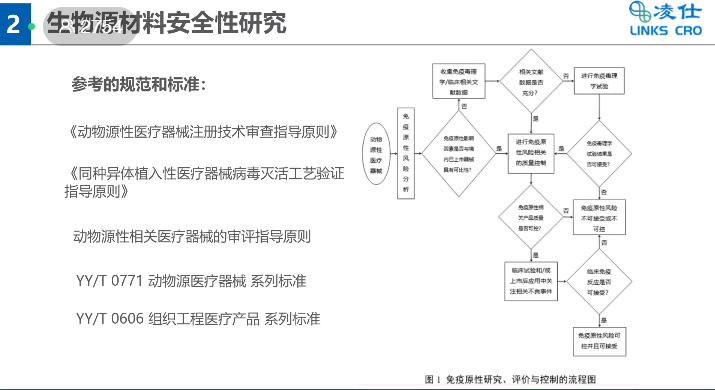
With the continuous growth of the Chinese medical device market and the ongoing updates to policies regarding the listing of innovative medical devices ,there have been many new requirements for the preparation of research materials for passive medical device registration. Brian Liu,manager of the LINKS Registration Department, shared experiences from the perspectives of regulatory requirements for registration application materials and common problems in the preparation of research materials. Detailed explanations were given on the regulatory basis, content of application materials, and research requirements for passive implantable devices, including studies on chemical and physical properties, biological characteristics, disinfection and sterilization, biological source material safety, and animal testing.
Additionally, all research throughout the lifecycle of medical devices is part of risk management and control. Mr. Liu pointed out that through risk studies, risks should be controlled, and the comprehensive residual risks of products should be kept within an acceptable range before approval can be granted.
Second Training丨Interpretation of Quality Management Norms for Medical Device Clinical Trials and Supervisory Situations
In March of last year, to further implement regulations and promote the development of the medical device industry, the National Medical Products Administration and the National Health Commission jointly issued the new “Quality Management Norms for Medical Device Clinical Trials,” which took effect on May 1 of the same year. This training invited experts of the Beijing Municipal Medical Products Administration to provide in-depth interpretations of the revised norms, discussing responsibilities and requirements from the perspectives of ethics committees, medical device clinical trial institutions, researchers, and sponsors. It was emphasized during the training that the requirements put forward by regulations are the basic regulatory requirements, and the purpose of regulatory inspections is to standardize clinical trials. Continuous learning and improvement in self-qualities and professional competence are necessary to promote the development of clinical trials and facilitate the introduction of more new medical devices to the market.
Third Training丨Selection and Reflection on the Clinical Evaluation Pathway for Medical Devices

Clinical evaluation is an essential part of the assessment of medical device safety and effectiveness, providing scientific evidence for product marketing. Different clinical evaluation pathways require varying amounts of work and time. Manager Ivy Huang from the LINKS Clinical Evaluation Department provided an in-depth explanation based on three aspects: clinical evaluation regulations, pathways, and considerations. For similar devices in the same category, with comparable technical and/or biological characteristics to the applicant’s product, including equivalent and comparable devices, the need for clinical evaluation depends on specific circumstances. For products not included in the exemption clinical evaluation catalog, it is necessary to clarify product characteristics, clinical risks, and existing clinical data, then determine whether clinical trials are needed based on the recommended clinical evaluation pathways in the “Medical Device Classification Catalog” subdirectories and the “Guiding Principles for Decision-Making on Whether to Conduct Medical Device Clinical Trials.”
The scene was hot and sparked intense discussions.
Summary
The online viewership of this training reached a remarkable 3,382 participants, and the live lectures received more than 26,500 likes. It not only discussed the latest changes in industry regulations and standards but also shared best practices and case studies. We would like to express our gratitude to all who participated and supported this training and sharing. We look forward to closer cooperation and deeper communication in the future, jointly promoting cutting-edge innovation and outstanding development in the medical device industry.
LINKS CRO
As a CRO company providing clinical trial services. LINKS CRO focus on innovation, high-risk implantation(intervention) medical device, with one-stop clinical trial solutions for the whole process of medical device research and development.The headquarters of LINKS is based in Shanghai, with subsidiary branches in Beijing, Shanghai, Guangzhou, and Shenzhen, in addition to ten regional offices. The current workforce comprises almost 200 employees, with technical personnel making up over 85% and dispersed across almost 30 cities nationwide.
Key Services include CRO, SMO, CER, Audit, Post-market research, core laboratory registration both domestically and overseas, innovation declaration, Expert Consultancy, and overseas enterprise agency services.
Key Indications include Cardiovascular, Neurovascular, Peripheral Vascular, Surgery Robot, Oncology, Ophthalmology, Medical Cosmetology, and other fields such as orthopedics. There are almost 300 projects consultations and services by LINKS each year.
